HIBC
This specification is mostly used in the field of medical devices. Connected barcodes can be used as well as separate primary and secondary codes. HIBC supports UDI identification. Code 39, Code 128, Datamatrix, QR code or Aztec can be used for barcodes.
Validation
When evaluating according to HIBC, all results are displayed with barcode information and evaluated identifier. The product identifier (the PCN for HIBC), the time stamp of the scan, the scanner ID as device identifier and the barcode type are displayed there. The overall result of the evaluation and the barcode content are below.
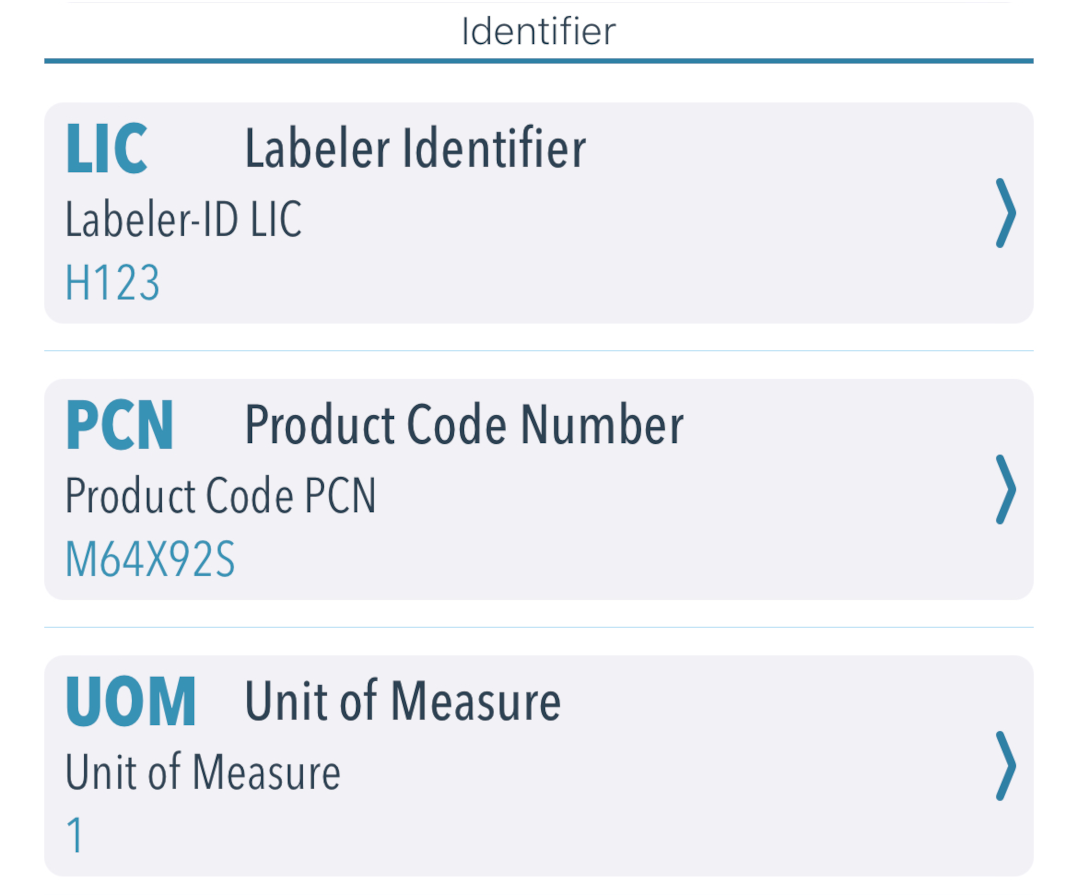
Then follows a list of evaluated identifiers. The output is similar to GS1, but HIBC barcodes have significantly fewer identifiers. The primary part consists of a manufacturer ID LIC, the product code PCN and the quantity index UOM. An expiry date MHD, a lot number LOT or serial number SER is printed in the secondary part.